
What is adult spasticity?
Spasticity is a condition that causes spasms and stiffness in your arms, legs, or both, interfering with movement and your ability to perform simple tasks, such as walking or dressing. The pain due to extreme stiffness and loss of mobility can become a turning point in your life.

What is adult spasticity?
Spasticity is a condition that causes spasms and stiffness in your arms, legs, or both, interfering with movement and your ability to perform simple tasks, such as walking or dressing. The pain due to extreme stiffness and loss of mobility can become a turning point in your life.
Signs & Symptoms
Are you experiencing the signs and symptoms of spasticity?
It can take many forms. These are some of the most common:
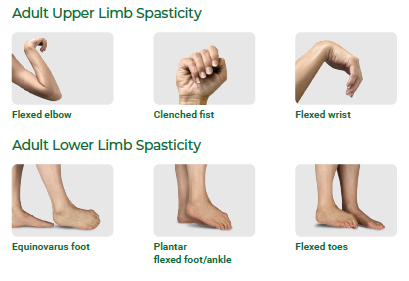
Don’t wait to talk to your doctor.
Your condition could worsen over time, so see your doctor as soon as you start having symptoms.
Causes of spasticity
What causes spasticity?

Spasticity can occur when the nerve cells that carry messages from your brain to different parts of your body are damaged. When this happens, the affected muscles in your limbs may receive the wrong signals. This can cause your muscles to contract or tense up, leading to stiffness and tightness.
Spasticity can occur because of different medical conditions or events that can damage nerve cells, including:
- A stroke
- A serious injury to the brain or spinal cord
- Diseases like cerebral palsy or multiple sclerosis

Developing spasticity can be a turning point in your life, because the extreme stiffness can be painful. It can also interfere with movement and your ability to perform simple tasks.
Be sure to tell your doctor about any and all symptoms you’ve been having, including:
- Muscle stiffness
- Pain
- Difficulty doing activities you were able to do before
If you think you might have adult spasticity, it’s important to identify it and discuss treating it with your doctor right away. Together, you and your healthcare team can create a plan that’s right for you.
In the United States, it is estimated that over 2.4 million adults experience some form of limb spasticity related to various medical conditions, including:
Stroke—1,495,000
Cerebral palsy—649,400
Multiple sclerosis—268,000
Managing Spasticity
Management of Spasticity
Treatment With Dysport®
- Dysport is injected into affected muscles to help lessen your symptoms.
- Dysport is a type of medicine that is injected directly into affected arm or leg muscles by a specialist. It helps to temporarily block signals from the brain that tell the affected muscles to contract or tighten, usually for months at a time.
- Response to treatment may be different for each patient, so be sure to talk to your doctor regularly during treatment.
- Talk to your doctor about your condition and whether Dysport may be an option.
How Dysport worked in clinical trials
For adults with upper limb spasticity, approximately 3 out of 4 patients had a response to treatment at week 4 as measured by a reduction in stiffness in the elbow, wrist, or finger muscles
For most patients, improvement was seen 1 week after treatment
For adults with lower limb spasticity, nearly half of patients had a response to treatment at week 4 as measured by a reduction in muscle stiffness at the ankle joint:
For most patients, improvement was seen 1 week after treatment
In these clinical studies, a majority of patients needed treatment again between 12 and 16 weeks.
The next Dysport treatment should not be given sooner than 12 weeks after the last Dysport treatment session. Your doctor will assess your spasticity at each treatment session and may adjust the dose and muscles injected.

